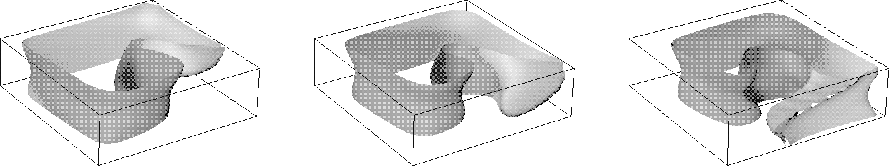
Figure 12: Spiral wave in the OGPV model in thee dimensions with rotational anisotropy of 3:1 velocity ratio and total rotation angle
The results described above are all for homogeneous isotropic 2D models; the real ventricle is three-dimensional, with an anisotropic fibre architecture, and inhomogeneous, both in the sense of presence of inexcitable obstacles (such as blood vessels) that can act to pin reentrant sources [42], and with gradients in excitation properties, the transmural endo-to-epicardial differences described by Antzelevitch [15, 16] and the base-to-apex differences that normally give the ventricular depolarisation and repolarisation the same polarity in the ECG. These anisotropies and inhomogeneities may mask the simple behaviours described above; in particular, the transmural rotational anisotropy cannot be be eliminated by a simple coordinate transformation.
In three-dimensional homogeneous media the generalisation of a spiral wave is a scroll wave, that can have an open linear or curved filament, or a closed filament that (in principal [43], but almost certainly not in the heart) can be knotted. Instead of considering motion of the spiral tip, we need to consider filament motion. The asymptotical approaches to the dynamics of the scroll filaments have been proposed [44, 45]; up to now only for non-meandering scrolls. Another approach to spiral and scroll waves motions comes from the application of the symmetries of the Euclidean group of transformations [46]; but this still has to be developed to account for curved scroll wave dynamics.
It is interesting that some important properties of scroll waves, for instance, the filament tension [45], that determines the stability of the scroll filament shape, can be found from 2D simulation, and so it is computationally feasible to approach this using the OGPV model. The practical interest in this quantity is that if the tension is negative, then in thick enough medium, the scroll waves will tend to multiply, and this might provide yet another theoretical scheme for the development of fibrillation.
A striking feature about the anatomical organisation of the ventricular muscle is the spiral organisation of the orientation of the muscle fibres on the epicardial surface. At any one point on the ventricular wall, as one penetrates the wall, the fibre orientation changes; there is a transmural rotational anisotropy. This rotational anisotropy not only may contribute to the formation of a re-entrant scroll wave [47], but can lead to spiral wave breakdown of a scroll wave [48]. This provides a resolution to the paradox [49] that numerical solutions, and experimental observations on thin ventricular slice preparations [50] demonstrate stable spiral while ventricular fibrillation is believed to be due to drift and breakdown of spirals [51].

Figure 12:
Spiral wave in the OGPV model in thee dimensions with rotational
anisotropy of 3:1 velocity ratio and total rotation angle ![]() ,
size of preparation
,
size of preparation ![]() .
.
Reproducing propagation in three-dimensional, biophysically realistic
cardiac wall models with rotational anisotropy is possible -- see
Fig. 12, but a systematic investigation is in practice at
the limits of available computing resources. Our preliminary approach
is to use restructurable grid schemes for solving the full biophysical
equations, with computations guided by phenomenology known from simpler
FitzHugh-Nagumo like caricatures. Within such a three dimensional model
it is possible, in principle, to incorporate transmural gradients in
the parameters of the excitation equations, with little increase in
computational load. Although we can simulate the transmural shape
changes in ventricular action potentials by scaling ![]() [18], the actual changes in ionic currents with position in the
ventricle are still being investigated, and so detailed simulations are
perhaps premature. What is feasible are preliminary computations that
simulate rather than reconstruct the changes in action potential shape,
to see if these changes in action potential shape, and their rate
dependence, have significant effects on propagation. Thus we are in a
position to move into three-dimensional computations of propagation in
currently realistic models of ventricular tissue, that include
biophysical, anatomical and histological detail. The modelling of
propagation phenomena in ventricular tissue and the whole ventricles is
feasible. The real test of these computational investigations will be
when they are validated against three dimensional visualisations of
propagating activity in real hearts, obtained via laser-mapping and
multiple electrode recordings.
[18], the actual changes in ionic currents with position in the
ventricle are still being investigated, and so detailed simulations are
perhaps premature. What is feasible are preliminary computations that
simulate rather than reconstruct the changes in action potential shape,
to see if these changes in action potential shape, and their rate
dependence, have significant effects on propagation. Thus we are in a
position to move into three-dimensional computations of propagation in
currently realistic models of ventricular tissue, that include
biophysical, anatomical and histological detail. The modelling of
propagation phenomena in ventricular tissue and the whole ventricles is
feasible. The real test of these computational investigations will be
when they are validated against three dimensional visualisations of
propagating activity in real hearts, obtained via laser-mapping and
multiple electrode recordings.